All published articles of this journal are available on ScienceDirect.
Human Brucellosis as an Epidemic Zoonosis in Zenica-Doboj Canton (Bosnia and Herzegovina) During 2008-2018
Abstract
Background:
Brucellosis is associated with people living in close proximity to their animals, where conditions for disease onset and spread exist. An epidemic of brucellosis in Bosnia and Herzegovina (B&H) has persisted since 2004. Zenica-Doboj Canton is one of the most affected areas.
Objective:
To investigate the epidemiological characteristics of human brucellosis from the year 2008 to2018.
Methods:
Data collected from paper-based patients/cases reported to the Epidemiology Department were analyzed.
Results:
After 2008, the annual number of patients diagnosed with brucellosis was decreasing, except in 2017 and 2018 with 20 and 35 cases, respectively. Within the 2008-2018 period, a total of 263 human brucellosis cases were recorded, decreasing from 102 (incidence of 44.7/100,000) cases in 2008 to three cases in 2012, but increased to 35 cases in 2018. Males were predominant, with a total of 205 (77.9%) cases. The mean age of the affected patients was 39.2 years; but the most affected age group was the 25-49 years age group with 117 (44.5%) cases. Most cases (151 cases, 66%) were reported during the period of March-July, and 242 (92%) cases were from the rural areas.
Conclusion:
With the implementation of the small ruminant vaccination program in 2009, the number of infected humans had declined, while brucellosis still remains.
1. INTRODUCTION
Brucellosis is zoonotic, food-borne, endemic, and (re)emerging infectious disease affecting wild and domestic mammals, especially cattle, small ruminants, and swine, causing abortion and reduced fertility [1]. The disease is transmitted to humans through the ingestion of contaminated dairy products such as raw milk and unpasteurized chees, as well as by direct contact, either cutaneous or mucous, with infected animals or biological materials including carcass, abortion products, and clinical samples [2-7]. Brucella and other zoonotic transboundary animal diseases (TADs) know no borders and can easily be transmitted to humans representing the classic example of spreading zoonosis as a result of population and animal migration [8-12]. The brucellosis is endemic in the Mediterranean basin countries, and Brucella melitensis infection, predominant biovar 3 [4, 13], is a regionally re-emerging infectious disease with an increasing number of human cases [7, 9, 13-16]. Moreover, some novel species were isolated from wildlife animals, some of them from humans [10].
The economy of Bosnia and Herzegovina (B&H) depends mostly on agriculture, employing about 20% of all workforces and contributing to 10% of the total gross domestic product (GDP) of the country. The national animal population consisted of 458,000 cattle, 1,125,000 sheep and goats, and 529,000 pigs [17]. Before the war (1992-95), B&H was a brucellosis free country and the one outbreak in 1985 was a consequence of an importation [3]. After the war, human brucellosis started to be recorded, probably as a consequence of the cattle donation to refugees who returned to the country that spread among livestock [2-4, 8-18]. Human brucellosis started with only one case in 1999 [2, 3], but spread among the human population to reach a peak of 1000 cases in 2008 [3, 17]. Therefore, a continuous animal vaccine program was implemented from 2009, the number of animal brucella cases started to decrease, but human brucellosis remained a serious threat in B&H.
The aim of this study was to investigate the epidemiological characteristics of human brucellosis in the period of 2008-20018 in Zenica-Doboj Canton, Bosnia, and Herzegovina.
2. METHODS
2.1. Study Setting
Zenica-Doboj Canton is situated in the central part of Bosnia and Herzegovina (B&H), in the Balkan Peninsula of southeastern Europe. The capital is Zenica, accommodating 30.4% of the canton population. Canton is 3904 km2, approximately 7.6% of the total area of B&H (51,129 km2). There are 12 municipalities with a total population of 364 433, approximately 10% of the B&H population is divided in the two distinct residential zones: the urban zone with 126,940 (34.8%), and a farming and agricultural rural zone with 237,493 (65.2%) inhabitants.
2.2. Study Design and Participants
A retrospective analysis of all human brucellosis cases between 2009 and 2018 was performed at the Epidemiology Department of the Institute for Health and Food Safety Zenica. Data were collected from paper-based patient case files kept at the Infectious Disease Department of the Cantonal Hospital Zenica.
The report contained the following data: hospitalization (notification) date (day/month/year), name, age, gender, place and area of residence (municipality/urban-rural), profession, employment status, firm/establishment, the month of disease onset, treatment before hospitalization at a general practice (GP), length of treatment before hospitalization (days), symptoms of the disease (fever, fatigue, chill, nocturnal sweat, dysuria, diarrhea, myalgia, weight loss, back pain, other symptoms), laboratory testing on Brucella, hospitalization (hospital, department, department of the second hospitalization if any - disease outcome, previous hospitalization for brucellosis), other household members with suspected brucellosis, results of laboratory analysis of household members, and epidemiology history.
Cases of brucellosis were diagnosed according to patient anamnesis, epidemiologic exposure, clinical manifestations, and positive results of the Rose Bengal plate test and confirmed with a positive result of Brucella spp. isolation from hemoculture and/or RVK and ELISA tests [19].
3. RESULTS
Between 2008 and 2018, a total of 263 cases of human brucellosis were recorded, with a decreasing pattern from 102 (38.8%) cases (incidence of 44.7/100,000) in 2008 to three (1.1%) cases in 2012, except the 35 (13.3%) cases in 2018. Males were predominant with 205 (77.9%) cases. The mean patient age was 39.2 years, and the most affected age was the 25-49 years group, with 117 (44.5%) cases (Table 1).
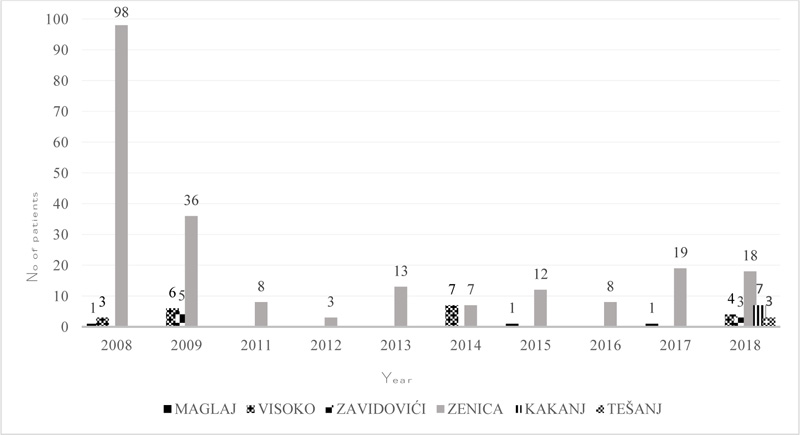
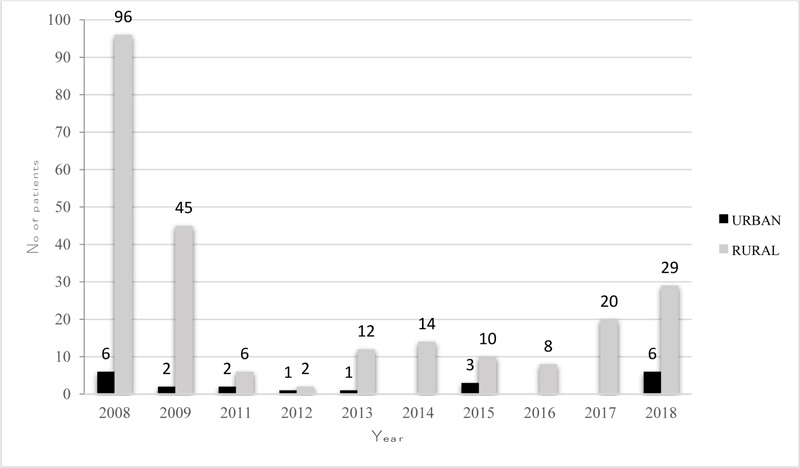
Patients from Zenica municipality were most frequently affected, with a total of 222 (84.4%) cases.. In fact, between 2011 and 2013, and in 2016 all human cases were from Zenica municipality while human brucellosis was recorded in the municipalities of Tešanj and Kakanj only in 2018 Fig. (1). Human brucellosis is noticeably high in the rural area Fig. (2) and during March-June, 150 (66%) cases were reported.
Fever, back pain, and nocturnal sweating were presented in more than 80% of the patients, while fatigue, chill, weight loss, myalgia, and headache were presented in a significant number of patients that ranged between 58.3 and 75.6% (Table 2).
| Characteristic | No (%) of patients | - | - | |||||||||||||
| 2008 | 2009 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | Total | ||||||
| Gender | ||||||||||||||||
| Male | 84 (82.4) | 38 (80.1) | 4 (50) | 2 (66.7) | 10 (76.9) | 9 (64.3) | 8 (61.5) | 4 (50) | 15 (75) | 31 (88.5) | 205 (77.9) | |||||
| Female | 18 (17.5) | 9 (10.9) | 4 (50) | 1 (33.3) | 3 (23.1) | 5 (35.7 | 5 (68.5) | 4 (50) | 5 (25) | 4 (11.5) | 58 (22.1) | |||||
| Age (groups) (years) | ||||||||||||||||
| 0-6 | 4 (3.9) | 2 (4.3) | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5) | 1 (2.9) | 8 (3) | |||||
| 7-14 | 11 (10.8) | 1 (2.1) | 0 | 0 | 2 (15.4) | 1 (7.1) | 3 (23.1) | 1 (12.5) | 1 (5) | 0 | 20 (7.6) | |||||
| 15-24 | 14 (13.7) | 3 (6.4) | 1 (12.5) | 2 (66.7) | 4 (30.8) | 3 (21.4) | 0 | 2 (25) | 2 (10) | 2 (5.7) | 33 (12.5) | |||||
| 25-49 | 48 (47.1) | 25 (53.2) | 5 (62.5) | - | 3 (23.1) | 7 (50) | 3 (23.1) | 2 (25) | 10 (50) | 14 (40) | 117 (44.5) | |||||
| 50-64 | 20 (19.6) | 15 (31.9) | 2 (25) | 1 (33.3) | 3 (23.1) | 3 (21.4) | 4 (30.8) | 2 (25) | 5 (25) | 13 (37.1) | 68 (25.8) | |||||
| 65-> | 5 (4.9) | 1 (2.1) | 0 | 0 | 1 (7.7) | 0 | 3 (23.1) | 1 (12.5) | 1 (5) | 5 (14.3) | 17 (6.4) | |||||
| Mean age | 35.5 | 40.5 | 39.1 | 33.6 | 37.6 | 37.2 | 45.6 | 39.2 | 39.2 | 48 | 39.2 | |||||
| Symptom1 | No (%) of patients |
| Fever | 156 (89.1) |
| Back pain | 156 (89.1) |
| Nocturnal sweat | 145 (82.8) |
| Fatigue | 135 (77.1) |
| Chill | 128 (73.1) |
| Wight loss | 119 (68) |
| Myalgia | 117 (66.8) |
| Headache | 95 (54.2) |
| Diarrhoea | 7 (4) |
| Insomnia | 12 (6.8) |
| Dysuria | 5 (2.8) |
| Nausea | 4 (2.3) |
| Orchitis | 4 (2.3) |
| Otitis | 4 (2.3) |
| Rash | 2 (1.1) |
| Chest pain | 4 (2.3) |
| Neck pain | 1 (0.5) |
| Cough | 4 (2.3) |
Rose Bengal test and hemoculture were positive in 233 (out of 263; 88.6%) patients. One patient died. A total of 240 (91.1%) patients were hospitalized. Suspected brucellosis within the same household was reported by 23 (8.7%) patients involving 91 (34.6%) household members in total (3.9 patients per household), and brucellosis was confirmed in 70 (76.9%) of them.
4. DISCUSSION
The number of brucellosis patients registered in FB&H had been increasing in the period of 2000-2008 [3, 8, 18], resulting in a significant increase in the incidence from 3.83/100,000 in 2004 to 33.4/100,000 in 2008 [3]. In the following years, the number of patients showed a decreasing pattern, probably because of the implementation of yearly mass vaccination of small ruminants, which started in 2009. However, after the initial decrease in the number of human cases between 2009 and 2016, an increase was noticed again in 2017 and 2018. Zenica-Doboj Canton was the most affected, going from only 15 patients in the 2000-2004 period [18] to 102 patients in 2008, thus, resulting in an incidence of 44.7/100,000. It is believed that the most important reason for this rise in human Brucella cases in the past two years is a delay of yearly decisions on animal vaccination (vaccination absence in the period of intense lambing). It is far higher compared to EU/EEA countries where brucellosis is well-controlled [20]. Within the same timeline (2017-2018), the number of reported human brucellosis cases in EU countries decreased to the lowest level since 2007: 381 cases were noticed in 28 EU/EEA countries (0.09/100,000 population), and 8 countries were brucellosis free. Among these countries, Greece had the highest rate of 0.87/100,000 population, followed by Italy and Portugal (both with 0.16/100,000 population), and Spain and Sweden (0.14/100 000 population) [20]. All 52 of the states in USA were brucellosis (cattle and swine) free (less than 0.25% herd infection rate) [21].
The main epidemiological characteristic of human brucellosis found in this study was in accordance with previously published data from B&H and other regions of the world as well: male predomination over female [2, 3, 5, 14, 18], 25-49 age group was the most affected [3,19], mean age of the patients was 39.2 years [4, 14]; patients were mostly from rural areas [2, 5], March-June was mostly the peak period [20] (coinciding with lambing season). Spring months were mostly reported as significant seasonal distribution of brucellosis in endemic regions (occupational), but in the brucellosis free countries, brucellosis is associated with summer months, which is connected with importation of the disease from endemic areas on the return of travelers [13, 20, 22].
The most reported disease symptoms found in this study include fever, back pain, and nocturnal sweating, which were similar to other reports [2, 4, 14]. The epidemiological characteristic of brucellosis in this study was expressed through 24 small family outbreaks involving 34.6% of ill patients, which is a usual finding [2, 14]. An appearance of infection in children (3%) is worrying. They were probably infected by in-house exposure from household members who were in contact with animals, as described previously [23].
An increase of prevalence of brucellosis is attributed to the movement of infected sheep or goats, which can contaminate pastures and spread brucellosis to other herds or areas [24.25], as well as direct occupational exposure to livestock in rural areas, and the consumption of unpasteurized dairy products in urban environments [3-7]. This movement is a major risk factor for the failure of brucellosis eradication programs, therefore, it is important to introduce animal surveillance alongside the borders in countries that are endemic for brucellosis [7, 26, 27]. It was found that knowledge of the mode of infection or the emphasis on pasteurization significantly reduced the risk of infection, thus confirming the importance of farmers/public health education [6, 24, 27].
Vaccination of sheep and goats is the mainstay of the current national brucellosis control and elimination strategies that are being implemented across Eastern Europe and Central Asia [15, 25], resulting in a substantial decline of small ruminant and human disease [15]. In some countries where small ruminant vaccination has been implemented, bovine brucellosis has also been reduced (e.g. Bosnia and Herzegovina, Kyrgyzstan), indicating that a greater proportion of bovine brucellosis is attributable to Brucella melitensis infection than it is commonly considered [15, 28]. Due to incomplete vaccination coverage, which was 70.22% in the 2009-2016 periods [29], and the presence of other factors, such as nomadic grazing, illegal animal trafficking, and lack of education, facilitate transmission among small ruminant as well as among B&H population.
The continuing existence of human brucellosis in B&H, with the potential for further increase in incidence, indicates that the control of brucellosis should join/integrate health professionals from the human and animal sectors and administrations. This effort extends beyond medical and veterinary duties and encompasses economic and even political factors [5]. However, the effectiveness of any strategy for the control or eradication of brucellosis must be supported by the farmers/animal owners; all resources should be available in advance, and all involved subjects should be well organized [20, 24, 25, 27].
CONCLUSION
This study has shown that following animal vaccination, human brucellosis is showing a decreasing pattern with occasional spikes, which might be effective in controlling using the one-health framework. B&H needs a brucellosis eradication program to prioritize and determine disease control strategy and prevention. This study underscores the importance of a holistic and multisectoral approach in order to successfully eradicate brucellosis. The One Health framework, recently established at our Institute, is an important step for better prevention and control of zoonoses, including brucellosis in Zenica-Doboj Canton.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical approval for this research has been obtained by Senad Huseinagić, MD, MA, Director of the Institute for Health and Food Safety Zenica (No 02-2509/18).
HUMAN AND ANIMAL RIGHTS
No human or animals were used in this research.
CONSENT FOR PUBLICATION
All patients participated on a voluntary basis and gave their informed consent.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
FUNDING
None
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
SU and MS conceived and designed the study; MS, DJ, and FB acquired the data; SU, MS, FB, FK, AI, and JD analyzed and interpreted the data; SU, FK, AI drafted the manuscript; SU, MS, FK, and AI critically revised the manuscript for important intellectual content; all authors gave approval of the version to be submitted; all authors agree to be accountable for all aspects of the work.


