All published articles of this journal are available on ScienceDirect.
Impact of Pregnancy on the Prognosis of COVID-19 in Women Hospitalized at the National Reference Center for Patients Infected with SARS-CoV-2 in a Resource-limited Country
Abstract
Aim:
The aim of the study was to reduce morbidity and mortality associated with COVID-19 in pregnant women.
Background:
Since the detection of the first case of COVID-19 on March 6, 2020, in Togo, pregnant women have received special attention due to their usual vulnerability to infection.
Objective:
The objective of this study was to evaluate the influence of pregnancy on the prognosis of COVID-19 in patients hospitalized in Lomé.
Methods:
This was an analytical cross-sectional study of women of childbearing age (15-49 years) admitted between March 22, 2020, and December 31, 2021, to the Lomé Commune Regional Hospital, a national referral center for COVID-19 patients.
Results:
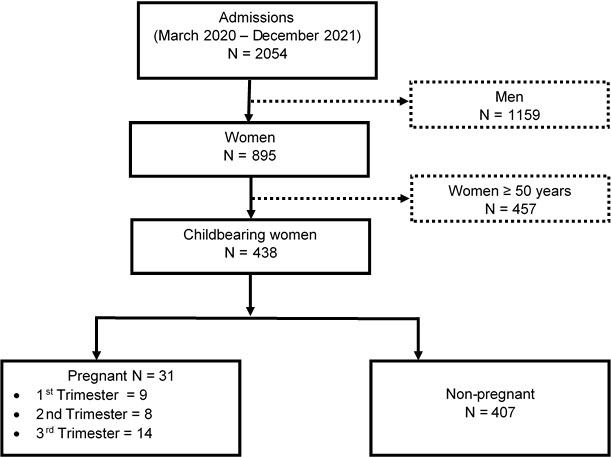
We registered 438 women of childbearing age, including 31 pregnant women (7.1%). Pregnant women were younger (28.8 years vs. 34.2 years, p = 0.001). Asthenia was more common in pregnant women (38.7% vs. 20.6%, p = 0.025), and SpO2 was lower (88.6% vs. 94%, p= 0.016%). Pregnancy was not associated with the occurrence of severe forms nor with prolonged hospitalization. Independent risk factors for mortality were 3rd trimester of pregnancy, mean age > 34 years, diabetes, HIV, and obesity.
Conclusion:
Most symptoms were similar to those observed in the general population. However, in addition to comorbidities, complications in the third trimester of pregnancy have worsened the prognosis for COVID-19. These results corroborate the observations made in the subregion. However, it is important to assess the effect of COVID-19 on pregnancy outcomes.
1. INTRODUCTION
1.1. Background
The latest epidemic that spread across the globe since the first cases was reported in China in December 2019. Coronavirus disease 2019 (COVID-19) has been the subject of unprecedented global mobilization due to its meteoric spread, forcing the WHO to declare it a public health emergency of international concern on January 30, 2020, and then a full-blown pandemic on March 11, 2020 [1, 2]. Although benign in its common form, the evolution of this infection can be severe, fraught with complications and death in elderly subjects or those with comorbidities [3, 4]. Early publications in small samples suggested that the prognosis of COVID-19 would not be worse in pregnant women [5, 6]. However, other studies have shown an increased risk of severe forms and death during pregnancy, but few of these studies were done in resource-limited countries of Africa [7-10]. Since the detection of the first case of COVID-19 on March 6, 2020, in Togo, specialized centers, such as the Lomé Commune Regional Hospital (CHR-LC), have been set up for the safe management of cases, along with other prevention and response measures against the epidemic. Since the means available are not sufficient to cover the entire population, the identification of vulnerable populations can allow a rational use of resources according to local realities. Pregnancy is a physiological condition usually associated with vulnerability to infections. But no studies have been devoted specifically to the impact of pregnancy on the prognosis of COVID-19 in Togo. This study aimed to determine the influence of pregnancy on morbidity and mortality associated with COVID-19.
1.2. Objective
The general objective of this study was to analyze the impact of pregnancy on the prognosis of COVID-19 in women hospitalized at the national reference center for the care of COVID-19 in Lomé, in comparison to non-pregnant women of the same age. Specific objectives were i) to describe the clinical presentation, ii) to compare mortality and severity of COVID-19 and the duration of hospitalization of pregnant versus non-pregnant women, iii) and identify the independent risk factor for mortality. We hypothesized that mortality would be significantly higher, about 20% in pregnant women, near 22.2% reported by Ngalame in Cameroon [7].
2. METHODS
2.1. Study Design
This is a retrospective cross-sectional study of all women of childbearing age admitted to hospitalization or confinement at the CHR-LC between March 20, 2020, and December 31, 2021. Data were retrospectively collected in February 2022.
2.2. Study Framework
The Lomé commune regional hospital is a general hospital that was refurbished at the beginning of the pandemic to care for patients with COVID-19. All confirmed cases of COVID-19, regardless of age, sex, or severity of disease, were admitted to this center. Indeed, it is the national reference center for the care of COVID-19 with a resuscitation unit, a conventional hospitalization unit, an isolation unit for confirmed asymptomatic cases, a dialysis unit, an imaging and medical radiography service, a current analysis laboratory and a pharmacy. Medical decisions were made by a multidisciplinary team of general practitioners and specialists (intensive care physicians, infectious diseases specialists, pulmonologists, internists, cardiologists, nephrologists, and neurologists). The Laboratory of the National Institute of Hygiene (NIH), the Laboratory of the Sylvanus Olympio University Hospital, the Biolim Laboratory of the University of Lomé, and the Mobile Laboratory of the Gnassingbé Eyadéma International Airport of Lomé served as a framework for PCR-SARS-CoV-2 tests. The Gynecology and Obstetrics Department of the Sylvanus Olympio University Hospital served as a reference for the care of pregnant women with obstetrical complications. All discharged patients had a systematic appointment for a check-up visit on the 30th day after discharge from the hospital.
2.3. Study Population
We systematically included files of all the women i) aged 15 to 49 years, ii) admitted to the CHR-LC during the study period, iii) having confirmed COVID-19 with a positive SARS-CoV-2 PCR test.
Pregnant women were defined as i) those with a pregnancy confirmed by rapid test and obstetric ultrasound in admission to CHR-LC ii) or in whom the pregnancy was already known and which was confirmed by an obstetric ultrasound from admission to CHR-LC, iii) or those who gave birth within 7 days before the diagnosis of COVID-19. Patients' database and medical records were confronted to reduce missing data, such as age, sex, gestational status, and outcome of hospitalization.
The severity of COVID-19 was determined according to the WHO classification [11]. We defined a « contact subject» as anyone who had contact with a confirmed COVID-19 case within the previous two weeks and a « suspect case» as anyone showing signs of COVID-19 according to the WHO case definition [11].
2.4. Data Collection
Data were collected retrospectively from the Excel database, registers and individual patient records. The main variable of interest was pregnancy. Confounding factors were age and the presence of comorbidities, such as high blood pressure, heart disease, diabetes, HIV infection, asthma, HBV infection, sickle cell disease, cancer, obesity, neurological impairment, gastritis, and gastroduodenal ulcer. The primary outcome was lethality, coded «death» or «survival». Secondary outcomes were COVID-19 severity and the length of stay.
2.5. Data Analysis
Data were analyzed with R 4.0.4 software. The qualitative variables have been presented in the form of numbers and respective percentages, and quantitative variables in the form of mean, standard deviation, or median. A comparative analysis was performed to look for a difference between the variables. The statistical tests used were Pearson's Chi-square test or Fisher's exact test for qualitative variables and Student's t-test or Wilcoxon test for quantitative variables. The significance threshold was set at 0.05. Univariate and multivariate logistic regression was performed to search for associated lethality factors. Variables statistically associated in the univariate analysis with a p ˂0.20 significance level were entered into the initial model. The top-down step-by-step procedure was used for the final model selection. It consisted of including all the selected variables in the initial model and then gradually removing the less significant variables. At each step, it was checked that there was no major confusion between the removed variable and those remaining in the model on the basis of changes in their Odds Ratio (OR) (tolerated variation: 20%) or even radical changes in their levels of significance. Multivariate analysis was used to estimate the adjusted odds ratio (aOR) and its 95% confidence interval for each retained variable. Once the final model was obtained, interactions between the different variables of the final model were sought by including interaction terms (product of the 2 variables concerned) in the model and checking their insignificance. The adequacy of the model was verified based on the R2 value.
2.6. Ethical Considerations
The study was conducted in accordance with good clinical practices and national and international recommendations for biomedical research. Patient confidentiality was respected by assigning anonymity numbers. Patient consent was not required, as the study was done retrospectively. However, we have obtained authorization from the Hospital Management for the investigation.
3. RESULTS
3.1. Study Population
During the study period, 2054 patients with confirmed COVID-19 were hospitalized, of whom 895 were women, i.e., an M/F sex ratio of 1.3. The frequency of pregnant women was 1.6% of all admissions, accounting for 3.5% of women and 7.1% of women of childbearing age. The distribution of women according to gestational status and age is shown in Fig. (1).
3.2. General Characteristics
The mean age of pregnant women was 28.8 ± 6.7 years, and that of non-pregnant women was 34.2 ± 9.2 years; p = 0.0012. Hypertension and diabetes were the most common comorbidities, followed by HIV infection and asthma, not correlated with gestational status (Table 1).
A small proportion of these women were tested for suspected COVID-19 symptoms, 9.7% in the « pregnancy » group and 6.1% in the « non-pregnancy » group, respectively. In 65.5% of all cases, data on the circumstances of the diagnosis were missing (Table 2).
| - | Pregnancy (N = 31) |
Without Pregnancy (N = 407) |
Total (N = 438) |
P-value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Diabetes | 0(0) | 43(10.6) | 43(9.8) | 0.059 |
| Hypertension | 3(9.7) | 62(15.2) | 65(14.8) | 0.600 |
| Cardiopathy | 0 (0) | 4 (1) | 4 (0.9) | 1 |
| HIV | 1(3.2) | 21(5.2) | 22(5) | 1 |
| HBV | 1(3.2) | 4(1) | 5(1.1) | 0.308 |
| Asthma | 1(3.2) | 23(5.7) | 24(5.5) | 1 |
| Sickle cell disease | 0 (0) | 13 (3.2) | 13 (3) | 0,612 |
| Cancer | 0 (0) | 11 (2.7) | 11 (2.5) | 1 |
| Gastritis/Peptic duodenal ulcer | 0 (0) | 17 (4.2) | 17 (3.9) | 0.622 |
| Neurological conditions | 0 (0) | 5 (1.2) | 5 (1.1) | 1 |
| Obesity | 1(3.2) | 17(4.2) | 18(4.1) | 1 |
| Other conditions* | 0 (0) | 22 (5.4) | 22 (5) | 0.388 |
| Circumstances | Pregnancy | Without Pregnancy | Total |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Unspecified* | 20(64.5) | 267(65.6) | 287(65.5) |
| Subject contact | 7(22.6) | 98(24.1) | 105(24) |
| Suspect | 3(9.7) | 25(6.1) | 28(6.4) |
| Travel | 1(3.2) | 17(4.2) | 18(4.1) |
| Total | 31(100) | 407(100) | 438(100) |
Unspecified*: missing data on the circumstances that led to the diagnosis of COVID-19.
3.3. Clinical Aspects
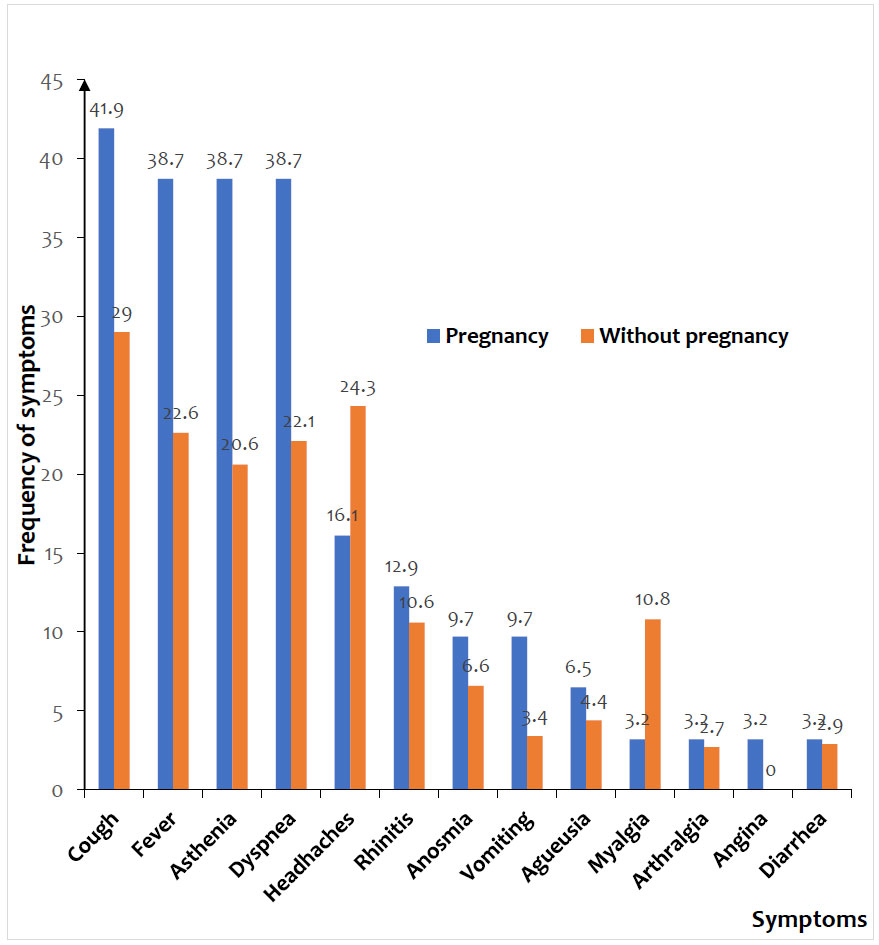
Cough (29.9%), fever (23.7%), headaches (23.7%), dyspnea (23.3%), and asthenia (21.9%) were the most common symptoms. The mean peripheral oxygen saturation (SpO2) was lower in pregnant women than in non-pregnant women (88.6% vs. 94.0%, p = 0.012). Similarly, asthenia (38.7% vs. 20.6%, p =0.025) was statistically associated with pregnancy (Fig. 2).
3.4. Outcomes
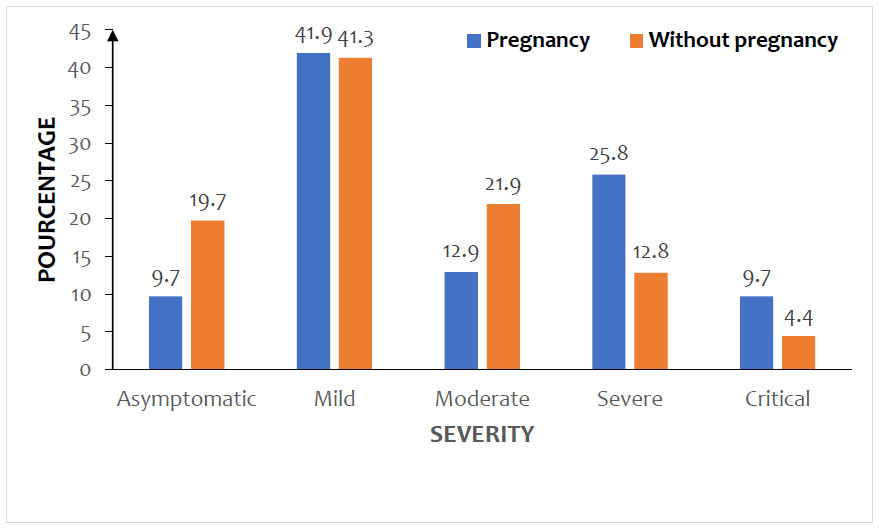
Severe and critical forms of COVID-19 were more observed in pregnant women than in non-pregnant women, with no statistically significant difference (p = 0.091). The distribution of women by COVID-19 severity and gestational status is shown in Fig. (3).
Non-obstetrical complications were mainly respiratory and neurological (Table 3).
Obstetrical complications were observed in 5 pregnant women (16.1%), all in the 3rd trimester of pregnancy. These were 2 cases of severe preeclampsia (6.4%), endometritis after caesarean section (3.2%), postpartum cardiomyopathy (3.2%), and overdue delivery (3.2%).
The mean length of hospitalization was 12.7 + 7.4 days for pregnant women and 13.8 + 7.2 days for non-pregnant women; (p = 0.432). We recorded 3 deaths of pregnant women, all in the 3rd trimester of pregnancy, and two of them after a caesarean section. The mortality rate of pregnant women was 9.7%, and that of non-pregnant women was 11.3% (p=1.09).
| - | Pregnancy (N = 31) |
Without pregnancy (N = 407) |
Total (N = 438) |
P-value |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| ARDS* | 2(6.5) | 5(1.2) | 7(1.6) | 0.081 |
| Renal | 1(3.2) | 1(0.2) | 2(0.5) | 0.882 |
| Neurological* | 0(0) | 7(1.7) | 7(1.6) | 1 |
| Pulmonary embolism | 0(0) | 2(0.5) | 2(0.5) | 0.791 |
| Other complications | 2(6.5) | 11(2.7) | 13(3) | 0.233 |
3.5. Risk Factors for Maternal Mortality
After bivariate analysis, age (OR = 1.86 ; 95%CI 1.08-2,5 ; p=0.0054), 3rd trimester of pregnancy (OR = 2.22 ; 95%CI 1.24-5.3 ; p=0.001), diabetes (OR = 2.2; 95%CI 1.09-3.45; p=0.006), HIV infection (OR=2.15; 95% CI 1.06-4.4; p=0.003), asthma (OR = 1.22; 95%CI 0.24-4.75; p=0.199) and obesity (OR=1.23; 95% CI 1.07-1.78; p=0.003) showed statistical association with a p ˂0.20 significance level.
But after multivariate analysis, asthma lost its significance and four variables remained statistically associated with the mortality; thus, the 3rd trimester of pregnancy (aOR = 2.08; 95% CI 1.17-3.57; p<0.001), mean age > 34 years (aOR=1.68; 95% CI 1.22-2.53; p<0.001), diabetes (aOR=2.05; 95% CI 1.49-2.26; p<0.001), HIV infection (aOR=1.83; 95% CI 1.17-3.36; p=0.002), and obesity (aOR=1.82; 95% CI 1.18-2.51; p=0.001) were significantly associated with increased mortality (Table 4).
| Factors | Mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| Bivariate Analysis | Multivariate Analysis | |||||||
| n/N | % | OR | 95% CI | P-value | aOR | 95% CI | P-value | |
| Age | - | - | - | - | 0.0054 | - | - | <0.001 |
| < 34 years | 11/204 | 5,4 | 1 | - | - | 1 | - | - |
| > 34 years | 38/234 | 16.2 | 1.86 | 1.08-2.5 | - | 1.68 | 1.22-2.53 | - |
| Pregnancy | - | - | - | - | 0.00065 | - | - | <0.001 |
| Non-pregnant | 46/407 | 11.3 | 1 | - | - | 1 | - | - |
| 1st Trimester | 0/9 | 0 | - | - | - | - | - | - |
| 2nd Trimester | 0/8 | 0 | - | - | - | - | - | - |
| 3rd Trimester | 3/14 | 21.4 | 2.22 | 1.24-5.3 | - | 2.08 | 1.17-3.57 | - |
| Hypertension | - | - | - | - | 0.773 | - | - | - |
| Yes | 11/65 | 16.9 | 1.56 | 0.26-2.28 | - | - | - | - |
| No | 38/373 | 10.2 | 1 | - | - | - | - | - |
| Diabetes | - | - | - | - | 0.0064 | - | - | <0.001 |
| Yes | 14/43 | 32.6 | 2.2 | 1.09-3.45 | - | 2.05 | 1.49-2.26 | - |
| No | 35/395 | 8.9 | 1 | - | - | 1 | - | - |
| HIV | - | - | - | - | 0.0031 | - | - | 0.002 |
| Yes | 9/22 | 40.9 | 2.15 | 1.06-4.4 | - | 1.83 | 1.17-3.36 | - |
| No | 40/416 | 9.6 | 1 | - | - | 1 | - | - |
| Viral Hepatitis B | - | - | - | - | 3.05 | - | - | - |
| Yes | 0/5 | 0 | 1 | - | - | - | - | - |
| No | 49/433 | 11.3 | 1.54 | 0.13-3.1 | - | - | - | - |
| Cardiopathy | - | - | - | - | 0.37 | - | - | - |
| Yes | 1/4 | 25 | 1.4 | 0.36-9.9 | - | - | - | - |
| No | 48/434 | 11.1 | 1 | - | - | - | - | - |
| Asthma | - | - | - | - | 0.199 | - | - | - |
| Yes | 3/24 | 12.5 | 1.22 | 0.24-4.75 | - | - | - | - |
| No | 46/414 | 11.1 | 1 | - | - | - | - | - |
| Sickle cell disease | - | - | - | - | 1.83 | - | - | - |
| Yes | 2/13 | 15.4 | 1.7 | 0.14-6.5 | - | - | - | - |
| No | 47/425 | 11.1 | 1 | - | - | - | - | - |
| Cancer | - | - | - | - | 0.85 | - | - | - |
| Yes | 3/11 | 27.3 | 1.32 | 1.07-1.95 | - | - | - | - |
| No | 46/427 | 10.8 | 1 | - | - | - | - | - |
| Gastritis/gastric ulcer | - | - | - | - | 1.33 | - | - | - |
| Yes | 2/17 | 11.8 | 1.17 | 0.21-8.75 | - | - | - | - |
| No | 47/421 | 11.2 | 1 | - | - | - | - | - |
| Neurological conditions | - | - | - | - | 0.69 | - | - | - |
| Yes | 3/7 | 42.9 | 1.5 | 0.04-25.06 | - | - | - | - |
| No | 46/431 | 10.7 | 1 | - | - | - | - | - |
| Obesity | - | - | - | - | 0.0012 | - | - | 0.001 |
| Yes | 6/18 | 33.3 | 1.23 | 1.07-1.78 | - | 1.82 | 1.18-2.51 | - |
| No | 43/420 | 10.2 | 1 | - | - | 1 | - | - |
3.6. Fate of the Children
We recorded two fetal deaths at 7 months of pregnancy: one mother was having severe ARDS and the second severe preeclampsia. Seven women (22.6%) delivered during the active phase of COVID-19, of which four had cesarean section. The indications for cesarean delivery were fetal malformation, pulmonary embolism, severe gravid hypertension, and overdue delivery.
The clinical evolution of the 7 newborns during the first 30 days of life was marked by the death of the malformed child on the 29th day. No complications were observed in the remaining 6 newborns.
4. DISCUSSION
4.1. Key Findings
Our study retrospectively evaluated the evolution of COVID-19 in 31 pregnant women, in comparison to 407 women of childbearing age.
The frequency of pregnant women was 1.6% of all admissions, accounting for 3.5% of women and 7.1% of women of childbearing age. Cough (29,9%), fever (23,7%), headaches (23,7%), dyspnea (23,3%), and asthenia (21,9%) were the most common symptoms in the two groups. Severe (25,8%) and critical (9,7%) forms of COVID-19 were more observed in pregnant women than in non-pregnant women, accounting for 12,8% and 4,4%, respectively. The mortality rate of pregnant women was 9.7% and that of non-pregnant women was 11.3%. The 3rd trimester of pregnancy (aOR = 2.08; 95% CI 1.17-3.57 ; p<0.001) was significantly associated with increased mortality. The other independent risk factors for mortality were mean age > 34 years (aOR=1.68; 95% CI 1.22-2.53; p<0.001), diabetes (aOR=2.05; 95% CI 1.49-2.26; p<0.001), HIV infection (aOR=1.83; 95% CI 1.17-3.36; p=0.002), and obesity (aOR=1.82; 95% CI 1.18-2.51; p=0.001).
4.2. Limitations of the Study
The retrospective nature of this study did not allow the consideration of certain specific pathology of pregnancy that could be the risk factor of severity for pregnant women, especially in the third trimester. In addition, it appears that the lack of knowledge regarding COVID-19 at the beginning of the pandemic contributed to the systematic admission of pregnant women, including those with moderate forms; this could lead to a selection bias. Finally, this retrospective study did not make it possible to determine the fate of pregnancy in a large proportion of our sample of pregnant women; this did not allow us to determine possible obstetric complications related to COVID-19 in both mother and child. As a result, the maternal mortality observed in our study may be underestimated. However, this study has the merit of assessing the impact of pregnancy on the severity of COVID-19 and on the mortality in the active phase of infection, while the first studies on the issue in sub-Saharan Africa focused on small samples, describing the clinical characteristics of pregnant women in a cosmopolitan hospital population where it is difficult to distinguish other prognostic factors for COVID-19, such as age, sex, and comorbidities. We chose to focus our study on women of childbearing age in order to harmonize the distribution of these confounding factors and to specifically analyze the influence of pregnancy.
4.3. Frequency of Pregnant Women
In our study, 7.1% of women of childbearing age were pregnant. This rate is comparable to the 6.6% reported by CDC in the USA on a large sample of women of childbearing age [8]. Pregnant women in our sample were significantly younger than non-pregnant women (28.8 ± 6.7 years vs. 34.2 ± 9.2 years; p = 0.001); this is a general trend in the African population where motherhood occurs at a relatively young age [5]. This young age has been mentioned as a protective factor for African populations against COVID-19 [12]. It seems that this young age contributed to a slight reduction in the mortality of pregnant women in our sample compared to non-pregnant women.
4.4. Symptoms
Only 9.7% of pregnant women and 6.1% of non-pregnant women were screened for suspicious symptoms, which is consistent with the benign nature of this infection in the African population [5, 13]. Respiratory signs, such as cough and dyspnea, were more frequent, but also general signs, such as asthenia, fever, and headache. The clinical aspects of COVID-19 are widely documented in the literature [5, 8, 13-16]. While some signs, such as asthenia, headaches and dyspnea, may be related to the physiological changes of pregnancy and delay consultation, others, such as cough and fever, should alert regarding the presence of an infection and raise suspicion of COVID-19. Mean oxygen saturation (SpO2) was significantly lower in pregnant women (88.6% vs 94.0% ; p = 0.012). A drop in SpO2 below 90% usually indicates severe impairment [11]. In Cameroon, 2 out of 3 pregnant women had SpO2 below normal [7]. In pregnant women, this decrease does not necessarily mean severe lung damage; in fact, other physiological factors can influence oxygen saturation during pregnancy [17]. However, these physiological changes may make the pregnant woman more susceptible to respiratory and thromboembolic complications [18].
4.5. Impact of Pregnancy and other Risk Factors on the Outcomes
Globally, pregnant women were admitted in a much more severe (25.8%) or critical (9.7%) clinical condition than non-pregnant women, who presented, respectively, 12.8% and 4.4% of severe and serious forms. However, this difference was not statistically significant, and the severity of the pregnant women’s clinical condition appears to be related to complications of the 3rd trimester of pregnancy. Some studies have shown a rarity of severe and critical forms in pregnant women, although the proportion of these severe forms is slightly increased compared to the general population [5, 14, 19]. Other authors have clearly identified pregnancy as a risk factor for severe forms [8, 9, 20, 21]. In both cases, the impact of various comorbidities on the severity of COVID-19 and on mortality in pregnant women was evident [4, 9, 10, 22]. In our study, these comorbidities also played a detrimental role in the severity of the disease and the mortality rate, independent of gestational status. Indeed, after bivariate and multivariate analysis, in addition to the 3rd trimester of pregnancy (aOR = 2.08; 95% CI 1.17-3.57; p<0.001), four other independent factors were significantly associated with increased mortality. These were mean age > 34 years (aOR=1.68; 95% CI 1.22-2.53; p<0.001), diabetes (aOR=2.05; 95% CI 1.49-2.26; p<0.001), HIV infection (aOR=1.83; 95% CI 1.17-3.36; p=0.002), and obesity (aOR=1.82; 95% CI 1.18-2.51; p=0.001). In a meta-analysis of the African series, Olumade and Col found a mortality rate of 5.6%, with wide variations ranging from 0.9% to 13.2% [13]. In contrast, our result is significantly lower than the 22.2% observed in a series of pregnant women in Douala [7]. In addition to age and the presence of comorbidities, obstetrical complications in the third trimester seem to be the determining factors of this excess maternal mortality [7, 15, 19].
4.6. Generalisability of the Study
Our observations are the results of data on a hospital population. They provide an overview of the hospital situation, especially in the active phase of infection, in resource-limited countries.
However, these results cannot be extended to the general population, given the benign nature of COVID-19, especially in Africa. Prospective studies with follow-up of large cohorts of women throughout the gestational and postpartum period will be more suitable.
CONCLUSION
The clinical manifestations in pregnant women were similar to those observed in non-pregnant women. The frequency of severe forms was slightly higher in pregnant women, with no statistically significant difference. Obstetric complications were observed in women during the third trimester of pregnancy; however, questions remain about the role of COVID-19 in the occurrence of these complications. Mortality and hospitalization length did not exhibit a statistically significant difference between the two populations. Regardless of gestational status, mortality was high in our sample, aggravated by advanced age and the presence of comorbidities, such as diabetes, HIV infection, and obesity, to which was added the impact of obstetric complications in the 3rd trimester. In light of these results, pregnant women should be screened for underlying pathologies and receive comprehensive care as part of their pregnancy follow-up in a specialized gynecology and obstetrics department. Finally, prospective studies are better suited to evaluate the medium- and long-term effects of COVID-19 on the mother-child pair.
LIST OF ABBREVIATIONS
| aOR | = Adjusted Odds Ratio |
| COVID-19 | = Coronavirus Disease 2019 |
| OR | = Odds Ratio |
ETHICAL STATEMENT
This is a retrospective study that was conducted after patients left the hospital. Patients’ consent was not required. Data were collected from archives, and patient confidentiality was respected by assigning anonymity numbers.
CONSENT FOR PUBLICATION
Patients’ consent was not required. Data were collected from archives, and patient confidentiality was respected by assigning anonymity numbers.
STANDARDS OF REPORTING
STROBE guidelines were followed
AVAILABILITY OF DATA AND MATERIALS
The data used for this study are available from the corresponding author [A. K.] upon request. The source of the data is the archive of patient’s files of Lomé Commune Regional Hospital Center, reachable by mail: pec_covid19_chrlc@yahoo.com
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


