All published articles of this journal are available on ScienceDirect.
Initial Finding of CYP9M10 Gene Haplotype in Culex Quinquefasciatus-Permethrin Resistant Isolate from Natural Population of Central Java Province, Indonesia
Abstract
Introduction:
Culex is a mosquito genus which widely distributed in the tropical, subtropical and temperate climates, both in urban and rural areas all over the world. Culex mosquitoes, especially Culex quinquefasciatus, are the main vector of filariasis. Thus, public health prevention is mainly directed to control these species and the chemical control is the most widely used approach. However, unfavourable effects of such control to the C. quinquefasciatus population have been known in the form of resistance and emergence of this resistance to various insecticides has been reported in many countries.
Methods:
The metabolic resistance in mosquitoes occurs through the expression of P450 gene family. One of the P450 families is CYP9M10 gene. Partial analysis of the CYP9M10 gene sequences C. quinquefasciatus mosquito reveals the differences between susceptible and resistant alleles. This study was started from the bioassay test of C. quinquefasciatus mosquitoes from five filariasis-endemic areas in Central Java and was continued on the molecular analysis of CYP9M10 gene.
Results:
The results of resistance analysis using bioassay test showed that the C. quinquefasciatus mosquito which has been isolated in Central Java showed high levels of resistance against permethrin 0.75% when mortality rates ranged from 4.8% to 21.6%.
Conclusion:
On the other hand, the molecular analysis revealed three types of haplotypes. The third haplotype had the highest frequency and it exhibited sequence pattern change in the CYP9M10 gene altering the susceptible strain into a resistant one.
1. INTRODUCTION
Culex is a mosquito genus which is widespread in tropical, subtropical and temperate climates, both in urban and rural environments around the world [1]. Culex mosquitoes, especially C. pipiens and C. quinquefasciatus, are the main vectors of filariasis in many regions of the world including the Middle East and Eastern Mediterranean countries [2-4]. Filariasis affects more than 25 million people with the disease in the genital area and more than 15 million people with lymphoedema. The distribution of patients is 65% lives in the region of Southeast Asia, 30% is in African region, and the rest is in the other tropical regions [5]. Japanese Encephalitis (JE) is also a disease which is transmitted by Culex mosquitoes and it is spread across South Asia, Southeast Asia, and Pacific [6]. The population at risk is estimated to reach 3 billion people and those live in the endemic countries of JE virus [7]. Culex is also responsible for the transmission of some viral diseases such as Rift Valley Fever [8], the West Nile virus [9-11], Saint Louis Encephalitis (SLE) and Eastern Equine Encephalitis (EEE) [12].
Filariasis is a disease which causes public health problems in Indonesia. According to the cumulative data up to the year 2016, there are 13,009 cases of filariasis clinical cases which spread over 278 of 514 Districts/Municipalities in Indonesia [13]. In Central Java, the number of filariasis cumulative cases by 2012 is 565 patients [14]. In 2013 and 2014, new cases of filariasis were found in 7 districts/municipalities, namely Semarang (11 cases), Demak (3 cases), Grobogan (1 case), the district of Jepara (2 cases), Pekalongan (2 cases), Sukoharjo (1 case), and Wonogiri (1 case) [15].
The distribution of Culex mosquitoes in various regions of the world, including Indonesia and its relationship with the incidence of filariasis has been reported [16]. The survey of vector density showed that Culex is the most dominant mosquitoes, both in Malaysia and Indonesia [17-19]. The density of C. quinquefasciatus mosquitoes reaches 5.91 mosquitoes/person/hour in the house and 4.75 mosquitoes/person/hour in the outdoors [20]. Culex mosquitoes have the highest infectivity against microfilariae compared with other genera [21]. Furthermore, the existence of C. quinquefasciatus mosquito habitat was associated with the incidence of filariasis [22].
Vector control is an important element of the strategy used to control vector-borne disease, and the chemical control is the most widely used approach [23]. The effectiveness of interventions using insecticide has been shown to reduce the incidence of malaria, and it is used in a variety of vector-borne disease control efforts in the epidemiology [24]. Something similar may also occur on the epidemiology of filariasis which is transmitted by Culex sp. The increase of insecticides utilization for vector control in the last decade has been associated with the increase of resistance due to the continuous pressure of the resistance genes. The data about vector resistance to insecticides is still limited and difficult to be found because many countries have not done any adequate routine susceptibility testing [5].
The resistance of Culex mosquito to various insecticides has been reported in many countries. The data from India indicates that C. quinquefasciatus is highly resistant to DDT and Malathion [25]. This mosquito is also resistant to Pyrethroids, Permethrin and Deltamethrin in Zambia [26]. Larvae of C. quinquefasciatus was resistant to Malathion and Permethrin in Kuala Lumpur [27], and it is also resistant to Pyrethroid in Zanzibar [28]. Our previously published paper found that this species also possess a high level of resistance against permethrin insecticide [29].
The mechanism of resistance to insecticides is very complex, including the behavioral change to avoid insecticides, physiological changes, reducing penetration and absorption, target site changes and metabolic resistance. Generally, the resistance in mosquitoes is associated with modification of the target site and metabolic resistance. The changes in the target site resistance include mutations that are resistant to chemical insecticides [30]. On the other hand, the metabolic resistance includes the subtler changes in the expression of a complex array of enzymes and detoxification pathways, the mechanism is still not well understood [31, 32]. Metabolic resistance occurs because of the increase of biological degradation to the insecticide, which is generally the mechanism occurs through overproduction of detoxification enzymes such as P450s, Glutathione S-Transferase (GSTs) and carboxyl/cholinesterase (CCE) [32].
P450s enzyme is expressed by CYP450 gene family. This gene has a role as the resistance marker to most of Pyrethroid Insecticides. Pyrethroid is the most widely known insecticide to be used in vector control. The increased activity level of CYP450 has been observed very often in the Pyrethroid resistance of malaria vector in Africa [33-35]. CYP450 is well-known as the main role in the resistance to Pyrethroid in Culex spp. [36, 37].
In the C. quinquefasciatus mosquitoes, four genes from CYP450 family have emerged as the candidates for resistance to permethrin i.e. CYP6AA7, CYP9J40, CYP9J34 and CYP9M10. The level of overexpression is closely related to the level of resistance in the mosquito laboratory strain of Alabama, USA [37-40]. The overexpression of CYP9M10 has been reported in the Culex-resistant mosquito laboratory strain in Saudi Arabia [41], which also metabolize permethrin and deltamethrin [39]. CYP9M10 is overexpressed in larvae of C. quinquefasciatus permethrin resistant in the laboratory strain [42]. CYP9M10 gene-resistant strain is expressed about 260-fold to pyrethroids compared with the susceptible strain [43].
Besides the data about the level of overexpression of detoxification enzymes, Single Nucleotide Polymorphisms (SNP) which encodes these enzymes have also been observed. Partial analysis of CYP9M10 sequences of C. quinquefasciatus mosquito revealed eight polymorphic locations distinguishing between susceptible alleles and resistant alleles [42]. Yet, there is no information related to the analysis of CYP9M10 genes in the C. quinquefasciatus mosquito population in the field. Thus, this study analyzed the site of CYP9M10 gene in the population of C. quinquefasciatus mosquitoes in the field that are resistant to permethrin.
2. MATERIALS AND METHODS
2.1. Mosquito Specimens and Bioassay Test
The specimens in this study are C. quinquefasciatus mosquitoes identified morphologically and genetically which are originally from 5 filariasis endemic Districts/Municipalities in Central Java in the year 2015 based on our previous report [29, 44]. Those areas are Semarang, Demak, Pekalongan, Jepara, and Grobogan. The adult mosquitoes in each district/municipality have been contacted using the insecticide of permethrin 0.75% for 1 hour with 24 hours holding period. The mosquitoes were distinguished between dead (susceptible) and alive (resistant), then continued for molecular detection of CYP9M10 gene in Eijkman Institute for Molecular Biology, Jakarta.
2.2. Polymerase Chain Reaction (PCR) of CYP9M10 Gene
Genomic DNA of C. quinquefasciatus was extracted using Chelex-100. Then, the DNA was amplified using Forward primers (9M-F): 5'- GAGGCGGAT CCAGTGTTAG -3' and Reverse primer (9M-R): 5'-TCAGTAG CTTCTTTAG GGATTATG-3' [42]. The detail of PCR reaction mixture can be found in our previously published papers [44]. The temperature for PCR conditions was 95ºC (5') pre-denaturation, continued for 40 cycles of 95ºC (30”), 50ºC (30”) and 72ºC (30”) and elongation period of 72ºC (5'). The PCR results were visualized using the electrophoresis method with 2% of agarose gel (2-gram agarose in 100 ml 1X TAE) in 10% ethidium bromide. Subsequently, the PCR products confirmed with gel electrophoresis were sent to bio service unit to be sequenced.
2.3. Sequencing Data Analysis
Improvement of sequencing results in the form of a DNA chromatogram was performed using Finch TV program. Blast identification method was performed at the site ncbi.glm.nih. DNA sequence alignment used Bio edit 7.1.9
Geographic location was determined using eTrex® 10 global positioning system (Garmin, USA) and was analyzed using Arc GIS 10.2
Statistical analysis of ӽ2 and graphic representation of calculation was performed by GraphPad Prism 7.0 program. Haplotype frequency calculations and haplotype visualization used were R (haplo.stats) and TCS 2.1 program, respectively.
3. RESULTS
3.1. The Result of Bioassay
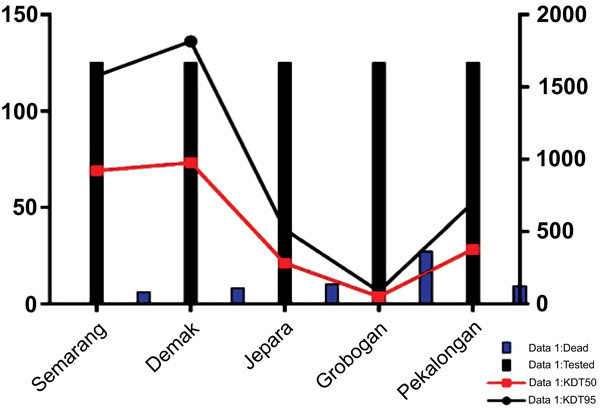
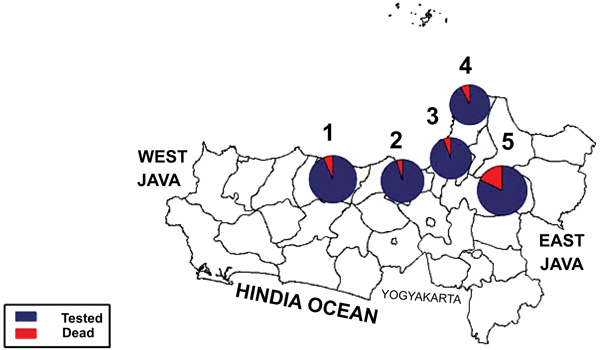
Fig. (1) showed a graphic representation of bioassay result indicating Grobogan was the highest number of died mosquitoes with the lower KDT50 and KDT95. However, all the sites were categorized to be highly resistant to permethrin. A mapping of the spread of the resistant can be seen in Fig. (2). Apparently, due to the most remote area, Grobogan possessed a relatively low level of resistant.
3.2. The Results of the Molecular Analysis of CYP9M10 Gene
Based on the previous report, there are four associated codons that discriminate between susceptible and resistant isolates. These codons are at position of 96, 134, 288 and 390. Our sequences have 99% similarities to reference sequence of SLAB (sensitive) and ISOP450 (resistant). No mutation detected at codon 96, only sensitive and heterozygote at codon 134 and 288 (Fig. 3). However, codon 390 exhibited all types of sensitive homozygote, heterozygote and resistant homozygote. Of each codon, there are 25 samples of mosquitoes, either dead (susceptible) or alive (resistant).
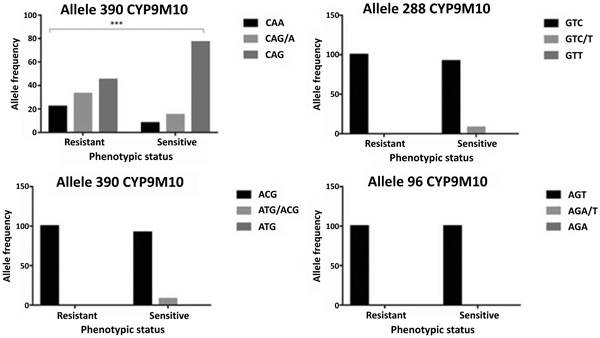
CYP9M10 gene at codon 390 showed polymorphism. The polymorphisms do not change the amino acid (silent mutation). There are three alleles at codon 390 i.e. CAA (wild-type), CAG (mutant allele) and CAA/G (heterozygote allele). Genotype frequencies of CAA, CAG / A, CAG and the allele frequency 390 CAA and 390 CAG respectively are 14%, 23%, 63%, 27% and 73% (Table 1). The tendency of changes occurred in the resistant allele (CAG). The allele frequencies in the resistant group are 56% for the CAG (mutant allele) and 44% for the CAA (wild-type). While on the susceptible group, the percentage increased to 85% for the CAG (mutant allele) and 15% for the CAA (wild-type). Additionally, there are a strong association of ӽ2 test result which showed P-value < 0.001 with a respective odd ratio as 4.706 (Table 1).
| CYP9M10 Phenotype |
Codon 96 | P-value of ӽ2 | Codon 134 | P-value of ӽ2 | Codon 288 | P-value of ӽ2 | Codon 390 | P-value of ӽ2 | OR CAA/CAG |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AGT | AGA/T | AGA | ACG | ATG/ACG | ATG | GTC | GTC/T | GTT | CAA | CAG/A | CAG | ||||||
| Resistant | 1,00 | 0,00 | 0,00 | - | 1,00 | 0,00 | 0,00 | - | 1,00 | 0,00 | 0,00 | - | 0,16 | 0,25 | 0,59 | 0.0004 | 4.706 |
| Sensitive | 1,00 | 0,00 | 0,00 | - | 0,90 | 0,10 | 0,00 | - | 0,90 | 0,10 | 0,00 | - | 0,1 | 0,2 | 0,7 | ||
Haplotype analysis showed that there were three types of haplotypes which were detected based on specific SNP. The calculations of haplotype analysis used R 3.2.1 program with haplo.stats packages. The three of haplotypes are ATTG (resistant strain), TCCA (susceptible strain) and TCCG (strain mixture). The frequencies of each haplotype are ATTG (8%), TCCA (36%), and TCCG (56%) (Table 2). It showed that the strained mixture and susceptible are dominant.
| Haplotype Code | Loc-1 | Loc-2 | Loc-2 | Loc-3 | Haplotype Frequency |
|---|---|---|---|---|---|
| ISOP450/ISOJPAL | A | T | T | G | 0.08 |
| SLAB | T | C | C | A | 0.36 |
| C | T | C | C | G | 0.56 |
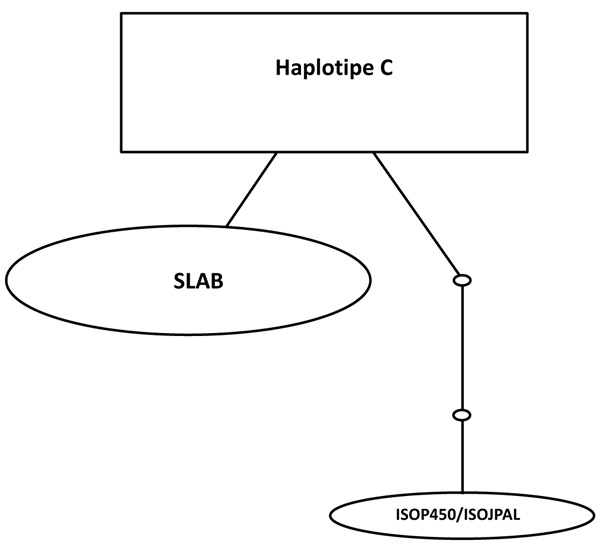
The relationships among each haplotype were analyzed using statistical haplotype kinship networks (Fig. 4). This haplotype kinship network statistics has a function for visualizing the pattern of linkages between each haplotype. Haplotype C which is the most commonly found haplotypes in this study indicates the closeness to the haplotype B / SLAB. While the haplotype A / ISOP450 indicates a distance of 2 small circles, which means that there are genetic distances related to the base substitution in specific SNP.
4. DISCUSSION
The results of resistance analysis using a bioassay test denote that C. quinquefasciatus mosquito originally from Central Java has a high level of resistance to permethrin 0.75%. These findings are different from the previous studies in the other countries which found that KDT50 ranged between 40-50 minutes [45] and 33.1 minutes [46]. High levels of resistance to permethrin among C. quinquefasciatus from Central Java, in contrast to some of the isolates from various countries exhibiting a vulnerable response to permethrin 0.75% [45, 47]. It is relatively higher than the isolates from Pathumthani, Nonthaburi and Bangkok of Thailand with a mortality rate of each permethrin with 67.4 ± 2.4%, 72.4 ± 0.4% and 80.6 ± 1.7% [48]. Although in some areas also found high levels of resistance in C. quinquefasciatus resistant to permethrin 0.75% i.e. the isolates of Benin with a range of 4-24% [49] and isolates of Baan Suan, Thailand, with an average mortality of 10.1% [50].
The high number of resistance of C. quinquefasciatus mosquito in Central Java can be the important information related to insecticide-based vector control program. In line with this study, some findings have described the distribution of vector resistance in Indonesia on Aedes and the degree of resistance is increasing [51-53]. In Central Java, a high resistance has also been reported in Aedes with mortality ranged from 1, 6-15, 2% and the KDT50 ranged from 120.2 to 1337.7 minutes [54].
The distribution of insecticide resistance to pyrethroid is various, it depends on the region. The four of the regions are the city of Semarang, Pekalongan district, Demak district and Jepara district, showed a very high level of resistance. This contrasts with the Grobogan district which showed a lower number than the other districts/cities. Geographically, Grobogan district is the most remote region compared to the other regions. However, it still shows a relatively high level of resistance. The phenomenon of the high level of resistance may be caused by an exposure of the local insecticides in dengue vector control program for a long time. Moreover, the breeding places of C. quinquefasciatus mosquitoes may also have been contaminated with insecticides [55].
Insecticides in Central Java have been very widely used with a high frequency. Moreover, the household insecticides and the repellant containing active compounds of pyrethroid have been increasingly used [54]. Interestingly, C. quinquefasciatus species may relatively develop the resistance process to the insecticide faster than other mosquitoes [56]. The close interrelatedness between the resistance and the intensity of insecticide exposure makes clear how the relationship between them is. So, it is necessary to evaluate the use of insecticides in a vector control program. Besides, it is needed for the society to understand the effects of the use of household insecticides.
CYP9M10 is one of gene families encoding P450s enzymes, and it is responsible toward the mechanisms of metabolic resistance to insecticides. The scientific publications about the mechanisms of metabolic resistance related to P450s enzymes are presented in the form of overexpression in the relevant genes, one of them is CYP9M10 [36, 38-43, 57]. The expression of the CYP450 gene is shown by the pure C. quinquefasciatus strain (ISOP450) of mating results between individuals exposed to permethrin over eleven generations [42]. Sequence analysis comparing the laboratory strain (SLAB) and ISOP450 have found five specific codons in exon and three in introns. We replicate a partial analysis using the field strain at four districts and one municipality in Central Java.
The sequence analysis results showed three out of four the analyzed exon sites showed that the isolates from Central Java are still susceptible to molecularly. It is because there are similarities to laboratory sequences (SLAB). However, this result is not in line with the high level of resistance in the bioassay test. It may occur due to a broad resistance mechanism which includes the reduction of penetration and absorption, the modification of target site (i.e. kdr gene mutation) and the complex mechanism of metabolic resistance [58]. Especially in the complex metabolic mechanism, it is not only influenced by a single gene, but also other genes included in the CYP450 gene family. It has a role in the expression of metabolic detoxification [59]. Notwithstanding, at codon 390, all three types of a sensitive homozygote, heterozygote and resistant homozygote were detected with statistically significant and odd ratio of 4.706 which strengthening the fact of haplotype analysis.
CONCLUSION
The study revealed three haplotype patterns between the reference sequence and the isolates from Central Java. Two of them are susceptible and resistant types. But, this study revealed that the third haplotype with the highest frequency which is a mix between susceptible and resistant alleles. This third haplotype may inform the existence of a pattern of sequence changes in the CYP9M10 gene from susceptible strain becomes resistant showing the association between the genotype and phenotype [60]. The data is supported by the variation on the composition of introns.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No animals/humans were used for studies that are the basis of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors wish to thank people who have consented to take a part in this study, to Eijkman Institute for Molecular Biology in Jakarta; Health Office of Semarang City, Grobogan District, Jepara District, Demak District and Pekalongan District, and those have helped.


